Measurement and analysis procedure used in aquaphotomics
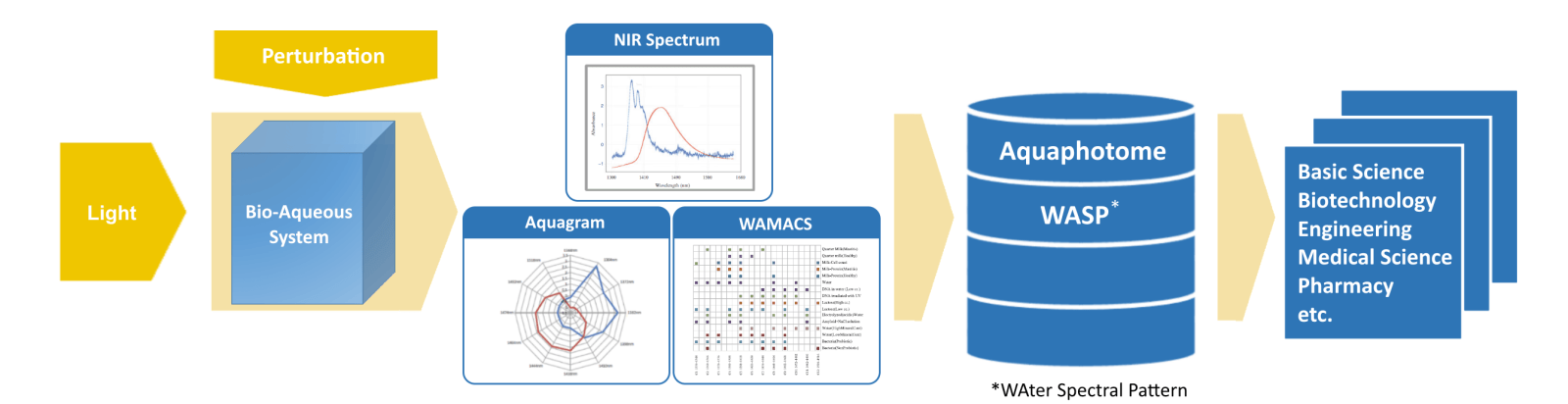
The method used in aquaphotomics consists of the following steps:
- Biological or aqueous system (e.g. as liquid in cuvette, or directly on surface/skin)
- Perturbation of the system with certain perturbation(s) (e.g. temperature, light, molecules, etc.)
- Acquisition of spectra (e.g. absorbance, transmission, for non-invasive dynamic studies VIS NIR range is most appropriate)
- Multivariate analysis of spectral variations to find and assign water bands per perturbation and create water spectral pattern WASP
- Visualize normalized WASPs in aquagram
- Add WASP to aquaphotome database
- Interpret and understand water functionality for use in biological, chemical, physical and more application areas
Data processing used in aquaphotomics
The improvement of detectors and the ongoing development of computers enable acquisition of immense number of spectra in real time as time series and under various possible perturbations. To appreciate and fully explore the richness of such copious spectral information, multivariate analysis is used. New methods have to be developed as very often, physical and chemical information are overlapped in the spectrum.
Aquaphotomics chemometric methods are continuously further developed, and water spectra under different perturbations are systematically acquired. Aquaphotomics already opens new horizons in understanding unknown phenomena in various fields. Once a large database of characteristic patterns of activated water bands, called the aquaphotome, has been acquired, they will be related to specific biological functions, (water) molecular structures, and used for understanding biology, chemistry and physics of water.
The 12 main WAMACS in the water first overtone (1300-1600nm)
Water bands have been identified in two ways, 1) experimentally and 2) by calculation of the overtone frequencies of published water bands in e.g. IR range, where fundamental water molecule vibrations have been assigned.
Experimentally, with aquaphotomics, 12 main WAMACS have been found ( C1-C12) in the water first overtone (1300-1600 nm) as a result of extensive study of various biological and aqueous systems. New WAMACS and WABs have been and will be discovered for more systems and perturbations.
WAMACS, which are the wavelength ranges where the variation of energy absorbance by water is largest in response to perturbations, are used as “hubs of information” to understand how water works.
At the moment, even without band assignment and understanding of water in contemporary way, WASPs can be used as holistic markers for system functionality.
However, in order to bridge the experimentally obtained results acquired through aquaphotomics approach to contemporary knowledge, it has been attempted to assign the WAMACS according to known water molecular structures. Conventionally, water molecular structures are characterized by hydrogen bonding, and water conformations, such as water dimers, trimers, superoxides, water solvation shells, etc.
So far, the WAMACS that have been found experimentally with aquaphotomics have shown to be consistent with published characteristic water bands for various water structures bands in IR range. Within one WAMAC more than one assignment is possible due to overlapping of vibrational modes in the overtone range. In the second and third overtone, similar spectral ranges for water are found, however there is even more overlap in those overtones.
Once a large database of characteristic water bands has been acquired, they can be related to specific biological functions and subsequently used for prediction, diagnosis, and understanding of biology, chemistry and physics of biological and aqueous systems.
In the following table the 12 WAMACS in the water first overtone are given with their tentative assignments based on the currently available resources . The list of contemporary water molecular assignments from literature and experimental evidence is continuously expanding. Similar tables can be made for the whole range of the EM spectrum. New views on water system physics will change the way we assign the WAMACS in the future.
| WAMACS | Range, nm | Assignment | Reference |
| C1 | 1336-1348nm | ν3, H2O asymmetric stretching vibration | (Ozaki 2002) |
| C2 | 1360-1366nm | Water solvation shell, OH-(H2O)1,2,4 | (Xantheas 1995, Robertson et al. 2003) |
| C3 | 1370-1376nm | ν1 + ν3, symmetrical stretching fundamental vibration and H2O asymmetric stretching vibration | (Ozaki 2002) |
| C4 | 1380-1388nm | Water solvation shell, OH-(H2O)1,4 and superoxide, O2-(H2O)4 | (Xantheas 1995, Weber et al. 2000) |
| C5 | 1398-1418nm | Free water and free OH- (S0) or trapped water | (Kaffka et al. 1990, Iwamoto, Uozumi, and Nishinari 1987, Kojić et al. 2014, Maeda et al. 1995) |
| C6 | 1421-1430nm | Water hydration, H-OH bend and O…O | (Tsenkova 2009, Williams 2009, Esquerre et al. 2009, Tsenkova et al. 2004) |
| C7 | 1432-1444nm | Water molecules with 1 hydrogen bond (S1) | (Segtnan et al. 2001b, Cattaneo et al. 2009, Ozaki 2002) |
| C8 | 1448-1454nm | ν2 + ν3, Water solvation shell, OH-(H2O)4,5 | (Cattaneo et al. 2009, Ozaki 2002) |
| C9 | 1458-1468nm | Water molecules with 2 hydrogen bonds (S2) | (Franks 1973, Tsenkova et al. 2004) |
| C10 | 1472-1482nm | Water molecules with 3 hydrogen bonds (S3) | (Franks 1973, Ozaki 2002) |
| C11 | 1482-1495nm | Water molecules with 4 hydrogen bonds (S4) | (Gowen et al. 2009, Segtnan et al. 2001b, Ozaki 2002) |
| C12 | 1506-1516nm | ν1, ν2, symmetrical stretching fundamental vibration, and doubly degenerate bending fundamental or strongly bound water | (Gowen et al. 2009, Segtnan et al. 2001a, Headrick et al. 2005) |
| Source: Modified from Roumiana Tsenkova, “Aquaphotomics: Dynamic spectroscopy of aqueous and biological systems describes peculiarities of water”, Journal of Near Infrared Spectroscopy 17, no.6:303-313 | |||
Reference
- Cattaneo, T. M.P., G. Cabassi, M. Profaizer, and R. Giangiacomo. 2009. “Contribution of Light Scattering to near Infrared Absorption in Milk.” Journal of Near Infrared Spectroscopy 17 (6):337-343. doi: .
- Esquerre, C. , A. Gowen, R. Tsenkova, C. O’Donnell, and G Downey. 2009. “Identification of water matrix coefficients in mushrooms (Agaricus bisporus) using robust ensemble of Monte Carlo uninformative variable elimination.” 14th International Conference on Near Infrared Spectroscopy, Bangkok, Thailand.
- Franks, F., ed. 1973. Water: A comprehensive treatise Vol. 3. New York: Plenum Press.
- Gowen, A. , R. Tsenkova, C. Esquerre, G. Downey, and C. O’Donnell. 2009. “Use of near infrared hyperspectral imaging to identify water matrix coordinates in mushrooms (Agaricus Bisporus) subjected to mechanical vibration.” Journal of Near Infrared Spectroscopy 17 (6):363-371. doi: 10.1255/jnirs.860.
- Headrick, J.M., E.G. Diken, R. S. Walters, N. I. Hammer, R. A. Christie, J. Cui, E. M. Myshakin, M.A. Duncan, M.A. Johnson, and K. D. Jordan. 2005. “Spectral signatures of hydrated proton vibrations in water clusters.” Science 308 (5729):1765-1769. doi: 10.1126/science.1113094
- Iwamoto, M., J. Uozumi, and K. Nishinari. 1987. “Preliminary investigation of the state of water in foods by near infrared spectroscopy.” Proceedings of the International NIR/NIT Conference, Budapest, Hungary.
- Kaffka, K.J., L. Horváth, F. Kulcsár, and M. Váradi. 1990. “Investigation of the state of water in fibrous foodstuffs by near infrared spectroscopy.” Acta Alimentaria 11 (2):125-137.
- Kojić, D., R. Tsenkova, K. Tomobe, K. Yasuoka, and M. Yasui. 2014. “Water confined in the local field of ions.” ChemPhysChem 15 (18):4077-4086. doi: 10.1002/cphc.201402381.
- Maeda, H., Y. Ozaki, M. Tanaka, N. Hayashi, and T. Kojima. 1995. “Near infrared spectroscopy and chemometrics studies of temperature-dependent spectral variations of water: relationship between spectral changes and hydrogen bonds.” Journal of Near Infrared Spectroscopy 3 (4):191-201. doi: 10.1255/jnirs.69.
- Ozaki, Y. 2002. “Applications in chemistry in near-infrared spectroscopy: Principles, instruments and applications.” In, edited by H.W. Siesler, Y. Ozaki, S. Kawata and H. M. Heise. Weinheim, Germany: Wiley-VCH Verlag GmbH.
- Robertson, W. H., E.G. Diken, E.A. Price, J-W. Shin, and M.A. Johnson. 2003. “Spectroscopic determination of the OH− solvation shell in the OH−·(H2O) n clusters.” Science 299 (5611):1367-1372. doi: 10.1126/science.1080695.
- Segtnan, V. H., Š Šašić, T. Isaksson, and Y. Ozaki. 2001a. “Studies on the Structure of Water Using Two-Dimensional Near-Infrared Correlation Spectroscopy and Principal Component Analysis.” Analytical Chemistry 73 (13):3153-3161. doi: 10.1021/ac010102n.
- Segtnan, V.H., S. Šašić, T. Isaksson, and Y. Ozaki. 2001b. “Studies on the structure of water using two-dimensional near-infrared correlation spectroscopy and principal component analysis.” Analytical chemistry 73 (13):3153-3161. doi: 10.1021/ac010102n.
- Tsenkova, R. 2009. “Aquaphotomics: dynamic spectroscopy of aqueous and biological systems describes peculiarities of water.” Journal of Near Infrared Spectroscopy 17 (6):303-313. doi: 10.1255/jnirs.869.
- Tsenkova, R.N., I.K. Iordanova, K. Toyoda, and D.R. Brown. 2004. “Prion protein fate governed by metal binding.” Biochemical and biophysical research communications 325 (3):1005-1012. doi: 10.1016/j.bbrc.2004.10.135.
- Weber, J. M., J. A. Kelley, S.B. Nielsen, P. Ayotte, and M.A. Johnson. 2000. “Isolating the spectroscopic signature of a hydration shell with the use of clusters: Superoxide tetrahydrate.” Science 287 (5462):2461-2463. doi: 10.1016/j.bbrc.2004.10.135.
- Williams, P. 2009. “Influence of water on prediction of composition and quality factors: The aquaphotomics of low moisture agricultural materials.” Journal of Near Infrared Spectroscopy 17 (6):315-328. doi: 10.1255/jnirs.862.
- Xantheas, S.S. 1995. “Ab initio studies of cyclic water clusters (H2O)n, n=1–6. III. Comparison of density functional with MP2 results.” The Journal of Chemical Physics 102 (11):4505-4517. doi: 10.1063/1.469499.